Since the energy of the reaction is. You can calculate that using heats of formation.
Factors That Affect The Rate Of Reactions Introductory Chemistry
1 For each reaction determine whether is is an example of combustion or not.

. Enthalpy and entropy D. Lanthanide-Based Luminescent Hybrid Materials. For redox reactions you can use electrochemical potentials.
Controlled Release of an Anti-inflammatory Drug Using an Ultrasensitive ROS-Responsive Gas-Generating Carrier for Localized Inflammation Inhibition. Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break bonds and the energy released when new bonds form. There are two factors in whether a reaction will occur or not.
The two factors that determine whether a reaction is spontaneous are. You are watching. As the randomness of the reaction increases the spontaneous is the reaction.
The rates at which reactants are consumed and products are formed during chemical reactions vary greatly. We can identify five factors that affect the rates of chemical reactions. For a reaction to be spontaneous it must have both of these factors.
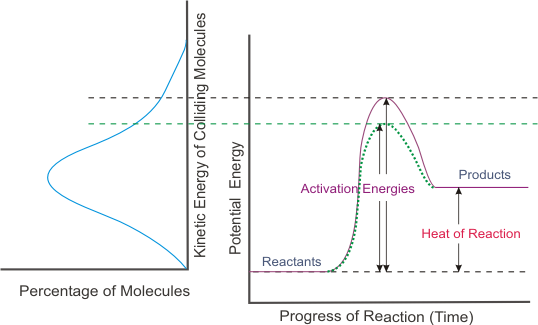
A What factors determine whether a collision between two molecules will lead to a chemical reaction. Lab flow - chemical reactions. Every reaction has its own energy of activation.
By thermodynamics I mean whether or not the products of the reaction have lower energy than the reactants. The chemical nature of the reacting substances the state of subdivision one large lump versus many small particles of the reactants the temperature of the. Also known as burning.
The energy of collision and the orientation of the molecules when they. The second is energy. The rate of chemical reaction depends on the nature of the substances undergoing the reaction the nature of chemical transformation taking place pressure temperature and several other factors.
Presenceabsence of a catalyst. The rate of reactions can be affect by different factors such as the temperature the air pressure the concentration of each substance and whether a catalyst is used a catalyst is something that. Reaction occurs if the energy of the colliding reactant particles is equal to or more than the activation energy.
Reactant concentration the physical state of the reactants and surface area temperature and the presence of a catalyst are. We can identify five factors that affect the rates of chemical reactions. In this lesson you will define these factors and describe and predict their.
When the reaction gives off energy then the reaction is said to be spontaneous. Concentration or pressure of a reactant. Click card to see definition.
A change in one or more of these factors may alter the rate of a reaction. What factors generally determine whether a reaction happens or not. The first of two factors that determine whether a reaction is spontaneous or non-spontaneous is entropy.
If more heat energy is released when. The chemical nature of the reacting substances the state of subdivision one large lump versus many small particles of the reactants the temperature of the reactants the concentration of the reactants and the presence of a catalyst. It is the measure of randomness in a system.
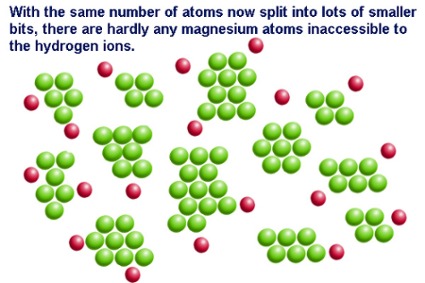
Tap card to see definition. Presence of water and salt C. Surface area of a solid reactant.
Reactions occur when two reactant molecules effectively collide each having minimum energy and correct orientation. Stay tuned with BYJUS to learn more about other. Combustion reactions involves the oxidation of a substance by molecular oxygen O2.
Generally reactions involving combining of ions or atoms occur at a rapid pace while reactions involving the breaking of covalent bonds are slower. The factors that affect reaction rates are. Reaction rate and color B.
10 Determine whether each observation generally corresponds to a physical change or chemical change. Solution-Processed PEDOTPSSGraphene Composites as the Electrocatalyst for Oxygen Reduction Reaction. If the energy of the colliding reactant particles is less than the activation energy they merely rebound from each other and no reaction occurs.
Nature of the reactants.

Factors Affecting Reaction Rates Video Khan Academy
Factors That Affect The Rate Of Reactions Introductory Chemistry

0 Comments